Pyrolysis of Plastic: A Potential Solution to the Plastic Pollution Crisis
What is the Challenge of Managing Municipal Solid Waste?
Municipal solid waste (MSW) management represents a persistent environmental, health, and economic challenge for developing countries, particularly due to escalating waste generation rates propelled by rapid population growth, urbanization, and elevated living standards. One potential solution to this challenge is pyrolysis of plastic or thermal decomposition of plastic. Thermal decomposition is a process that breaks down plastic waste into its constituent materials, such as oil, gas, and char. These materials can then be used to produce new products, such as fuels, chemicals, and building materials.
Thermal decomposition is a sustainable and environmentally friendly way to manage plastic waste, and it has the potential to reduce the amount of plastic that ends up in landfills and incinerators. In addition, the shift of millions of individuals from rural to urban regions across the globe has compounded this issue, leading to about 1.3 billion tons of MSW annually in these developing regions.
How Does Plastic Waste Factor into the MSW Problem?
One potentially underutilized aspect of MSW is its potential as a resource for biomass, recyclable materials, and energy, which can generate revenue if managed efficiently. Despite this, numerous municipalities lack the technical and financial capabilities to implement environmentally friendly waste disposal strategies. Glaring examples of this issue are in most developing nations, where MSW management difficulties are mirrored, with an alarming waste generation rate estimated to double by 2033.
Plastic waste is a significant portion of MSW, owing to its widespread use in various products. This is because of plastic’s durability, lightweight nature, and cost-effectiveness. However, managing plastic waste presents challenges, as most plastics are non-biodegradable and persist in the environment for hundreds of years.

What are the Limitations of Conventional Recycling Methods?
Conventional recycling methods offer a limited solution, recycling only a small fraction of total plastic waste. As a result, alternative approaches, such as thermal and catalytic pyrolysis of plastic, gasification, and plasma arc gasification, are increasingly considered for plastic waste recycling. For example, pyrolysis processes convert plastic waste into liquid oil, solid residue, and gases, albeit with some limitations, such as temperature dependency and potential contaminants in the produced oil.
What is Pyrolysis of Plastic and How is it Used in Recycling Plastic Waste?
Pyrolysis is a recycling method that transforms organic polymers into liquid oil, char, and gases using high-temperature thermal decomposition. This process has been experimented with using varying temperatures, typically between 300-900°C, with optimal plastic waste results around 500-550°C. The process speed, or heating rate, has also been varied, with researchers using rates like 4°C/min, 20-25°C/min, and 10°C/min.
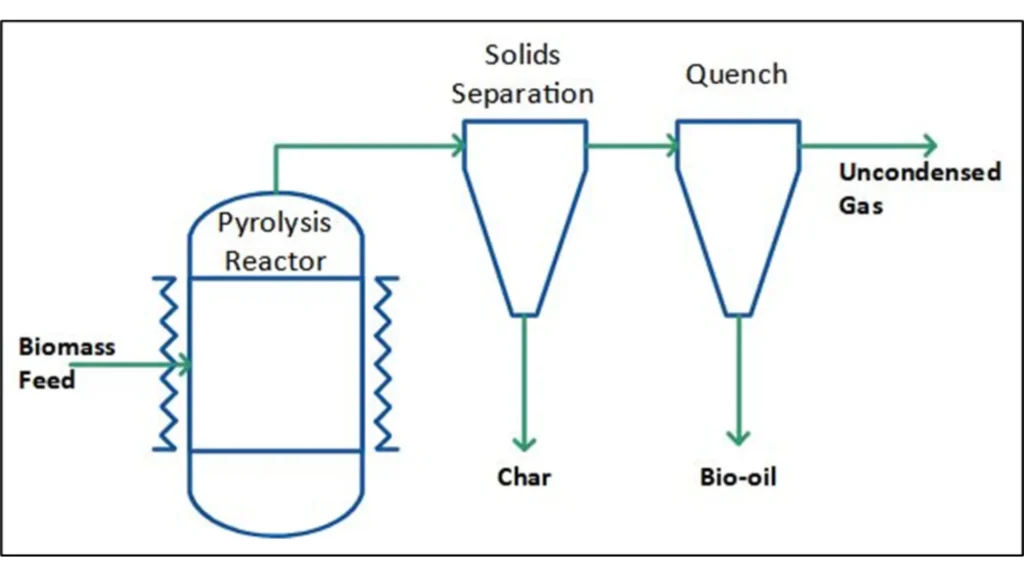
How Does Catalytic Pyrolysis Enhance the Recycling Process?
The duration of the pyrolysis process, or retention time, is another variable researchers have been exploring. Some studies have used retention times ranging from 0-15, 15-30, and 120 minutes. Other studies have set fixed times, like 40-70 minutes, 120 minutes, or 45 minutes.
One study conducted pyrolysis on HDPE at various temperatures between 400-550°C. They found that the retention time decreased as the temperature increased, going from 760 minutes at 400°C to 54 minutes at 550°C. Despite these findings, there has still potential for additional optimization, with possibilities for reducing process temperature and retention time. Pyrolysis can be conducted through both thermal and catalytic methods.
What is the Role of Catalysts in Pyrolysis?
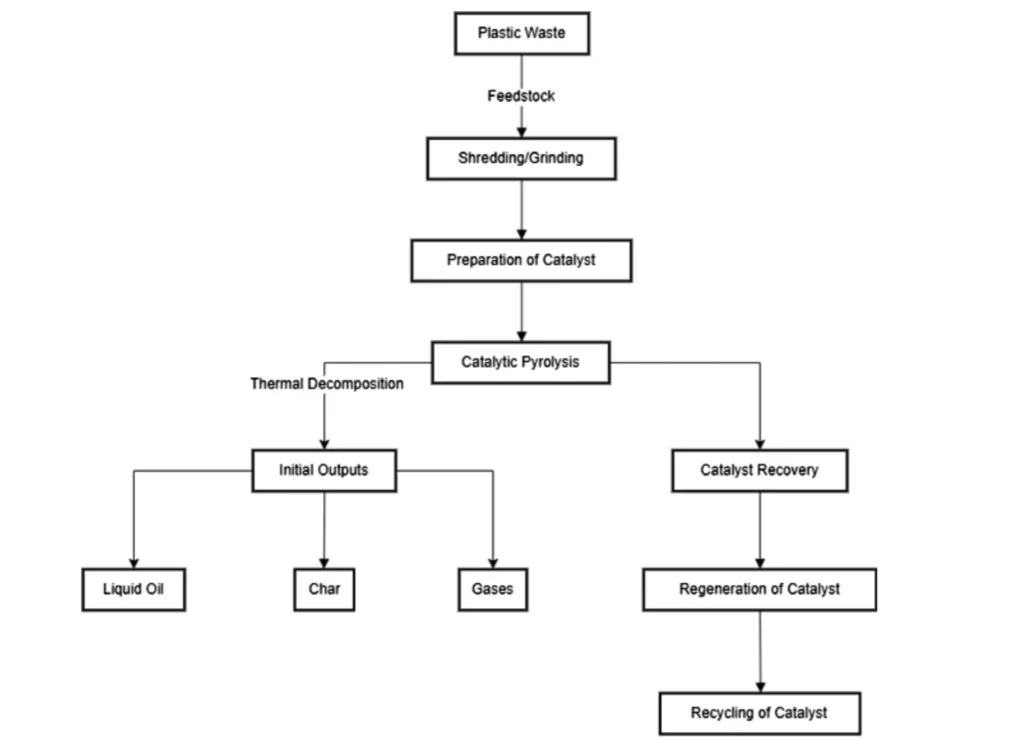
Catalytic pyrolysis of Plastic was developed to address these limitations. This process involves using various catalysts to enhance the quality of the liquid oil produced and reduce the energy required. Catalysts can also reduce impurities in the resulting oil and increase the cracking reaction rate to produce more gases.
What are the Potential Outputs from Pyrolysis and Their Applications?
Even though research is progressing in exploring different catalysts and their roles in pyrolysis of plastic, comprehensive reviews on the achievements, challenges, and future directions in the catalytic pyrolysis of plastic waste are still lacking. In addition, the scientists delve into catalyst reforming and developing new, more cost-effective catalysts to enhance the economic viability and sustainability of the catalytic pyrolysis process.
What is the Potential Future of Catalytic Pyrolysis in Waste Management?
Recognizing these drawbacks has led researchers to explore the concept of catalytic pyrolysis of plastic. This approach involves using catalysts to enhance the process, improving the quality of liquid oil and gases, and reducing the process temperature and time required. In addition, it is a testament to the power of scientific innovation in optimizing resource-intensive processes.
Choosing the right catalyst for the breakdown of plastic waste is crucial in pyrolysis. Therefore, efforts have been made over the past decade to improve the efficiency of this process by modifying catalysts. These modifications can be achieved through various methods, such as acid leaching, thermal treatment, and wet impregnation.
How Do Catalyst Characteristics Impact Pyrolysis Outcomes?
The specific characteristics of a catalyst, like its BET surface area and acidity, directly impact the pyrolysis outcomes. For example, a catalyst with a high BET surface area and acidity yields more gas, reducing liquid output and vice versa. Furthermore, microporous catalysts exhibit similar behavior, whereas macro-porous catalysts increase liquid oil and char yield with less gas production.
What Catalysts are Involved in Pyrolysis and How Do They Work?
Several catalysts, including ZSM-5, HZSM-5, Fluid Catalytic Cracking (FCC), Al2O3, Red Mud, and Natural Zeolite (NZ), have been identified as instrumental in this process. These catalysts, each unique in their properties, have been employed by researchers globally, marking a significant stride in the evolution of pyrolysis of plastic. Advancements such as catalyst reforming, which involves doping metals like Ni, Co, Mo, and Zn into acidic catalysts, further enhanced the catalytic activity.
How Can Catalysts be Improved for Enhanced Performance?
Heating catalysts, for example, at a temperature of 500°C for three hours, can eliminate volatile impurities, thus increasing their catalytic activity. Using substances like HCl, acid leaching also purges impurities and improves the catalyst’s acidity, enhancing its overall performance. The wet impregnation technique is another popular method for creating diverse catalysts.
What Are the Effects of Catalyst Reforming?
Reformed catalysts show a dual effect: metallic sites quicken hydrogenation and dehydrogenation reactions, while acidic sites accelerate the isomerization reaction. Combining zeolite with elements such as zinc can boost the adsorption capacity of the catalyst and restrict zeolite pores. This restriction creates an effective sieving effect, allowing only small molecules to pass through. Changed zeolites usually have a particle size in the micron range, with an internal surface area over 99% of the total surface area. As a result, nearly all the active sites are located inside the pores, enhancing their adsorption and purification capacity.
What Is the Potential of Reformed Catalysts?
It has been noted that adding elements like Cu to Al2O3 increases the overall surface area, pore volume, and pore size. Using these reformed catalysts can elevate the overall efficiency of catalytic pyrolysis and improve the quality of the products. Despite these advances, challenges and opportunities remain for further improvements in the catalytic pyrolysis of plastic waste.
What Is the Role of Pyrolysis in Waste Transformation and Energy Generation?
Researchers have explored and analyzed the diverse aspects of this complex procedure, focusing primarily on its parameters, advantages, the role of catalysts, and future opportunities. Pyrolysis of plastic, a thermal degradation process, facilitates the transformation of plastic waste into valuable products such as liquid oil, char, and gases, each possessing its unique potential applications.

How Does Pyrolysis-Derived Liquid Oil Contribute to Energy Generation?
Liquid oil, derived from pyrolysis of plastic, offers an alternative energy source. It has been reported that plastic waste can be converted into liquid oil between 78-84% by weight, showcasing its impressive efficiency. This pyrolytic liquid oil possesses properties akin to conventional diesel, reinforcing its utility for energy. It can be used for diverse heating applications and even as a transportation fuel, significantly advancing the renewable energy landscape.
The fuel can also be blended with conventional diesel in varying proportions and used in diesel engines. While blending affects engine performance and exhaust emissions, studies show that 20% pyrolytic oil and 80% conventional diesel do not significantly alter engine performance. Interestingly, as the ratio of pyrolytic liquid oil increases, fuel consumption decreases, though this effect is mitigated by the lower calorific value of the pyrolytic liquid oil compared to conventional diesel.
What Are the Environmental and Energy Applications of Char?
Char, another byproduct of the pyrolysis process, also holds the potential for environmental and energy applications. Though produced in smaller quantities than liquid oil and gases, char presents unique uses. It has been found to possess significant amounts of fixed carbon, volatile matter, and moisture content, with a small proportion of ash. As a result, it has a high heating value (HHV) and can heat water efficiently.
In addition, char can aid in heavy metal adsorption from municipal and industrial wastewater and remove toxic gases. It can also be used as a feedstock for activated carbon or as an energy source for boilers. Finally, char’s characteristics can be enhanced by thermal or steam activation, decreasing sulfur content and making it more environmentally friendly.

What Are the Potential Applications of Gases Produced Through Pyrolysis?
Lastly, gases produced through the pyrolysis of plastic comprise methane, hydrogen, propane, propene, ethane, ethene, butane, and butene, presenting many potential applications. Interestingly, these gases hold high calorific values, indicative of their potential as an energy source. For instance, gases from agricultural plastic waste and those produced from polypropylene (PP) and polyethylene (PE) have high HHVs, demonstrating their energy generation capability.
The produced gases can be used in boilers for heating or gas turbines for electricity generation without flue gas treatment. Moreover, based on their composition, valuable compounds such as 1-butene and isoprene can be recovered and used in tire production. In addition, propene and ethane can be isolated and used as chemical feedstocks to produce polyolefins, further expanding their utility.
Why Is Pyrolysis Important in the Energy Sector?
The products of the pyrolysis of plastic have substantial value. For instance, the liquid oil and gases produced have a high heating value (HHV), making them a promising alternative energy source. As we grapple with dwindling fossil fuel reserves and the escalating global energy demand, the significance of such renewable energy sources cannot be overstated. If harnessed efficiently, these materials derived from waste could help ease energy poverty and drive energy transitions, particularly in developing regions.
How Can Char Contribute to Mitigating Environmental Pollution?
The char byproduct of pyrolysis of plastic also boasts significant utility. Possessing a high Brunauer–Emmett–Teller (BET) surface area, char can serve in various environmental applications. Notably, it can facilitate the adsorption of heavy metals and other pollutants from wastewater and air, offering a viable solution for mitigating environmental pollution. With the escalating pollution levels worldwide, such environmentally friendly char applications could contribute to global sustainability goals.
What Are the Challenges and Intricacies in the Pyrolysis Process?
However, the pyrolysis process is not without its intricacies. A myriad of factors, including temperature, retention time, feedstock composition, and the use of catalysts, can significantly influence the process’s efficiency and the quality and yield of the end products. Thermal pyrolysis of plastic, for instance, yields lower-quality liquid oil and requires high temperature and retention time, posing substantial operational challenges.
How Does Catalyst Modification Enhance Pyrolysis?
The role of the catalyst in pyrolysis of plastic is not static. Researchers continuously explore catalyst modifications such as thermal treatment, acidic treatment, and metal doping through wet impregnation, aiming to augment the catalytic activity by improving the catalyst characteristics. Using Natural Zeolite (NZ) as a catalyst is acquiring momentum because of its easy availability and economic feasibility.
What Is the Future of Catalytic Pyrolysis for Waste Management?
In conclusion, pyrolysis of plastic, especially catalytic pyrolysis, emerges as an innovative, sustainable, and promising route for managing plastic waste. The explorations and advancements in this field offer encouraging prospects for waste management, energy generation, and environmental protection. While challenges persist, continuous research and development promise to further refine and optimize this process, leading us toward a more sustainable and resource-efficient future.
To Cite this article:
‘A.S. Nizami. Plastic’s New Purpose: Unpacking the Potential of Pyrolysis. 2023. Publication & Data. Green Flagship.’

